In the following compressibility factor Z vs pressure graph at 300
4.6 (258) In stock

In the following compressibility factor Z vs pressure graph at 300 K, the compressibility of CH 4 at pressure
In the following compressibility factor Z vs pressure graph at 300 K- the compressibility of CH 4 at pressure -200 bar deviates from ideal behaviourA- The molar volume of CH 4 is less than its molar volume in the ideal stateB- The molar volume of CH 4 is same as that in its ideal stateC- Intermolecular interactions between CH 4 molecules decresasesD- The molar volume of CH 4 is more than its molar volume in the ideal state

The van derWaals constant a for different gases are given below Gas aatmlit2mol2 O2 136 N2 139 CH4 225 NH3 417 The gas that can be most easily liquefied is

Solved Molar volume of the gas calculated from ideal gas

Compressibility factor (gases) - Knowino

WPILARIVIANN ZU 60. ollowing compressibility factor (2) vs pressure graph 300 K, the compresability of Cheatre 200 bar deviates from ideal behaviour because Compressibility Factor (2) Ideal gas 02 0 200 600

How is matter classified at micro level

Modus Operandi for Practice: A Comprehensive Study Package on Atomic Structure and the Gaseous State, PDF, Spectroscopy
Which gas shows the maximum deviation from ideal gas, CO2 or NH3? Why? - Quora
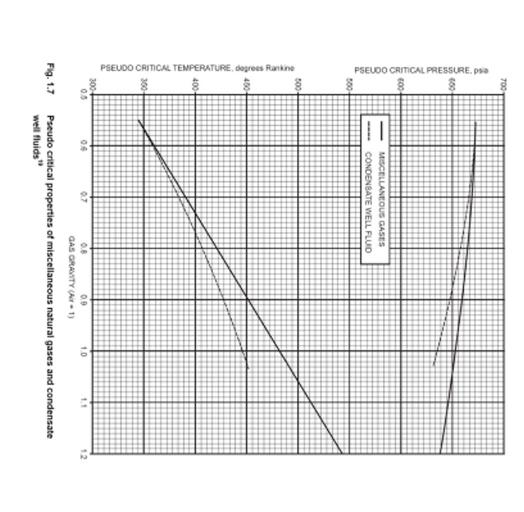
Solved Calculate the gas deviation factor, gas formation
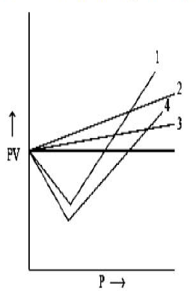
Assuming that the behaviour of ammonia is correctly described by the van der Waals equation near the critical point and knowing the critical molar volume of 072500 mol L1 and critical temperature of 4053 K determine the critical pressure of ammonia

2nd Year Holiday Assignment, PDF, Tangent

plotting - How to plot Compressibility factor Z vs Pressure P using ParametricPlot? - Mathematica Stack Exchange
Compressibility factor Z for sub-critical pressures in a 'one-cell' formula for excel spreadsheets
Two extensions of the compressibility factor Z correlation (sub-critical pressure region)
What is the compressibility factor (Z) for 0.02 mole of a van der Waal
 Vintage New With Tags Flexees Floral Deluster Firm Control Long
Vintage New With Tags Flexees Floral Deluster Firm Control Long- AQUA Faux Leather Bralette Top - 100% Exclusive
- STANFIELD'S LIMITED LONG JOHN UNDERWEAR, FR, CSA, 5.7 CAL/CM SQ, NAVY, SZ 3XL, 5 OZ, KERMEL/LENZING JERSEY - Arc Flash & Flame-Resistant Long Underwear - NVTFR21-125-3XL
 Skort playsuits PrettyLittleThing
Skort playsuits PrettyLittleThing Underwired Non-Padded Bra
Underwired Non-Padded Bra- Fajas de Manta Naksak - Faja doble uso: Prenatal y Post-Parto


