Ideal–Universal Gas Law
4.8 (725) In stock
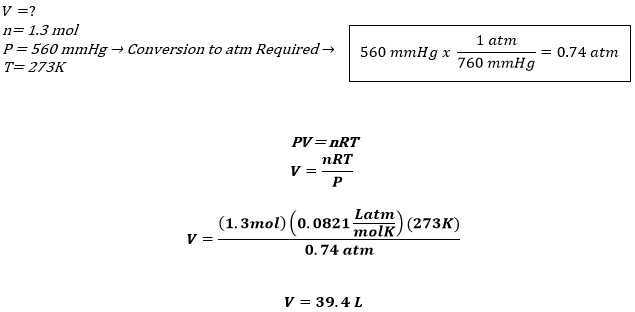
Definition: The Universal or Ideal Gas Law describes the relationship between all four properties (pressure, volume, number of moles, and temperature) as well as a gas constant called “R.” NOTE: The Ideal Gas Constant “R” has constant a value of 0.0821 L.atm/mol.K Relation: The relation between pressure (P) volume (V), number of moles (n) and…
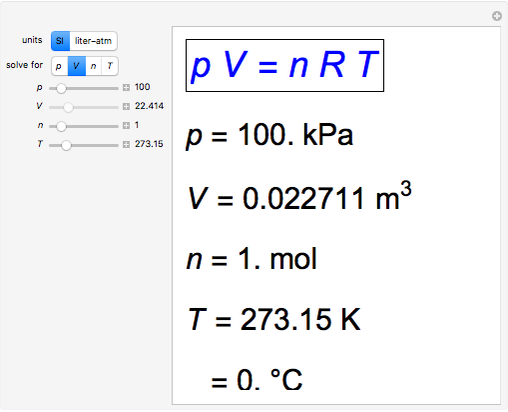
Ideal Gas Law Solver - Wolfram Demonstrations Project
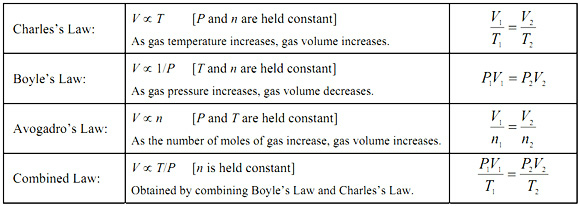
What does the ideal gas law allow a scientist to calculate that the other gas laws do not?
Ideal Gas Law - Wyzant Lessons

Physical States of Matter

SI Units

physical chemistry - What is the relation between universal gas constant R and amount of substance n? - Chemistry Stack Exchange

MathType on X: The gas constant “R” is defined as the Avogadro constant “NA“ multiplied by the Boltzmann constant “k”. It is mostly known for appearing in the ideal gas law and

ANESTHESIA EQUIPMENT AND GAS LAW REVIEW - ppt download
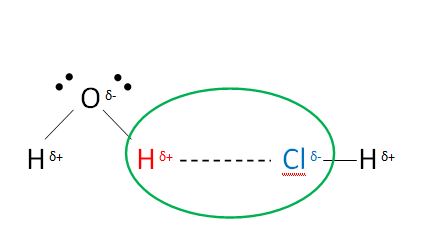
Intermolecular Forces of Attraction

Metallic Character

Lecture 4 Gas Laws and Relations, PDF, Gases
Ideal Gas Law: Statement, Characteristics, Formula & Problems
The Ideal Gas Law: Density - Video Tutorials & Practice Problems
Predicting Gas Pressure Using the Ideal Gas Law - dummies
6.5.3 Ideal Gas Equation, AQA A Level Physics Revision Notes 2017
 Nike WMNS Yoga Luxe Strappy Cami Top (Peach) DA0732-288 – Allike Store
Nike WMNS Yoga Luxe Strappy Cami Top (Peach) DA0732-288 – Allike Store- Buy Zivame Beautiful Basics Padded Wired 3/4th Coverage T-Shirt Bra - Spectra Green at Rs.450 online
 Slit Skirt With Shirred Waistband Beginner Friendly Instant Download PDF Sewing Pattern EU 32-54 US 0-22 Sizes Sun Bell Skirt
Slit Skirt With Shirred Waistband Beginner Friendly Instant Download PDF Sewing Pattern EU 32-54 US 0-22 Sizes Sun Bell Skirt Women's Hiking Pants Convertible Scout Safari
Women's Hiking Pants Convertible Scout Safari- Lululemon Ribbed Train Bra Medium Support, C/d Cups In Rainforest
 Men's Leggings with front gusset -Charcoal- App Bittersweet – Appalachian Bittersweet
Men's Leggings with front gusset -Charcoal- App Bittersweet – Appalachian Bittersweet

